Epileptic seizures are short disturbances of brain activity due to abnormal behavior of cells in the brain. Seizures, which originate from one part of the brain are called focal seizures. Commonly it is assumed that when excitatory and inhibitory synaptic inputs in the group of neurons become unbalanced, the network becomes hyperexcitable. On the other hand under conditions of blocked chemical synaptic transmission seizure activity may persist and even spread. This paradox might be linked to the fact that seizures are accompanied by significant changes of concentrations of ions (Na+, K+, Cl-, Ca2+, H+ and HCO3-) in the extracellular and intracellular space. These changes shape, in turn, neuronal excitability. Multiple feedback loops between ions and neuronal activity are hard to study experimentally. Therefore it is not easy to determine whether ion changes lead to seizures or vice versa. Physiologically realistic computational models that take into account neurons and their environment with dynamic changes of ionic concentrations may help to solve this puzzle. The goal of the project is to develop highly realistic computational model of neurons embedded in the ionic environment with activity-dependent changes of ion concentrations. The model is used to investigate the link between ionic dynamics and seizure patterns observed experimentally.
The project is carried out in collaboration with the Lab from Carlo Besta Neurological Institute in Milan, Italy, where experimental setup of the whole brain in vitro was developed. This unique in the world preparation gives an advantage of performing intracellular and ion-selective recordings together with extracellular local field potentials (LFP) measurements in the intact, whole brain networks. Application of proconvulsive drugs leads to seizure patterns that bear close resemblance to human temporal lobe seizures recorded with invasive intracranial electrodes. For this reason, the whole brain in vitro preparation is considered an animal model of human focal epilepsy.
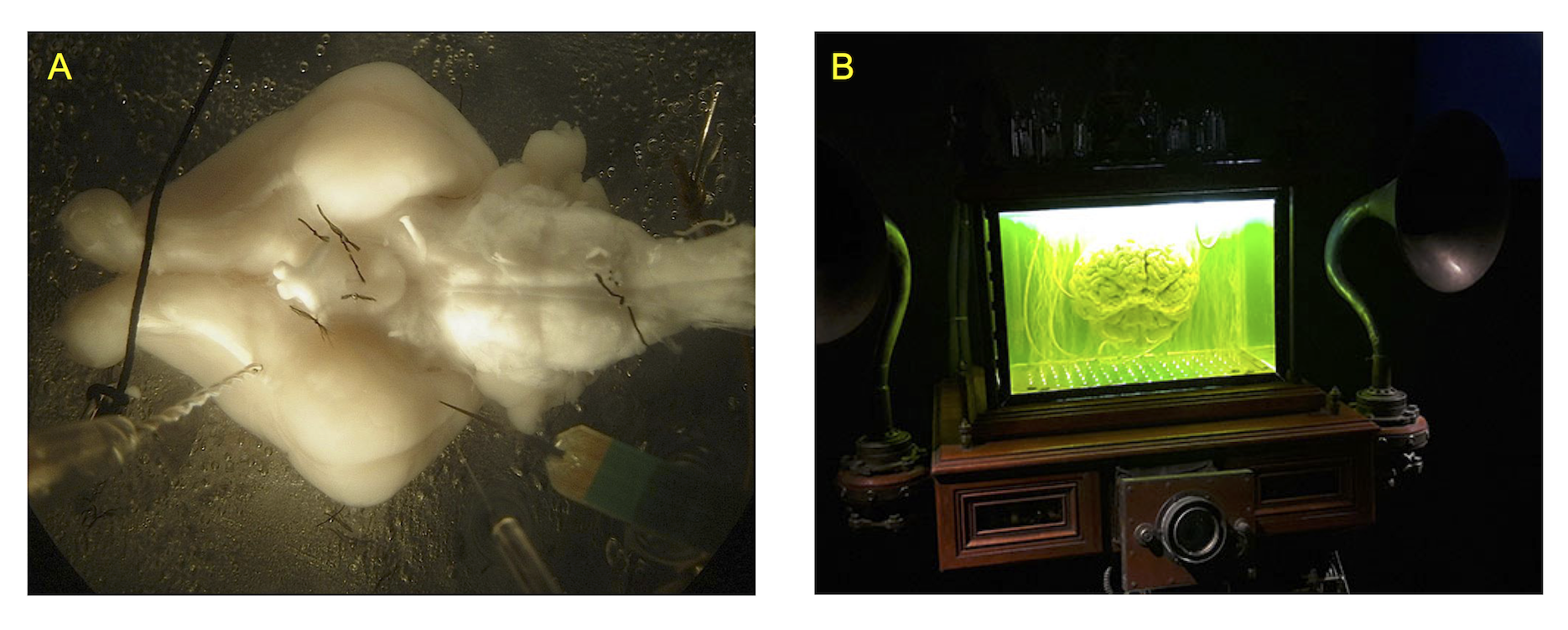
Figure 1. A. Whole guinea pig brain in vitro experimental setup. B. Brain in the aquarium in the sci-fi movie The City of the Lost Children (1995).
Currently, our computational model consists of five multicompartmental cells - four pyramidal neurons and an inhibitory interneuron - embedded in the common extracellular space surrounded by a bath, where concentrations are at their normal physiological levels. Neurons are connected by chemical excitatory (AMPA) and inhibitory (GABAA) synapses. Ionic dynamics of K+, Na+, Ca2+ and Cl− is incorporated and changes in their intracellular and extracellular concentrations are computed. Concentration changes are dependent on a number of mechanisms such as: active and passive membrane currents, inhibitory synaptic GABAA currents, Na+/K+ pump, KCC2 cotransporter, glial K+ buffering, radial diffusion between extracellular space and bath, longitudinal diffusion between dendritic and somatic compartments and volume changes of intra- and extracellular space.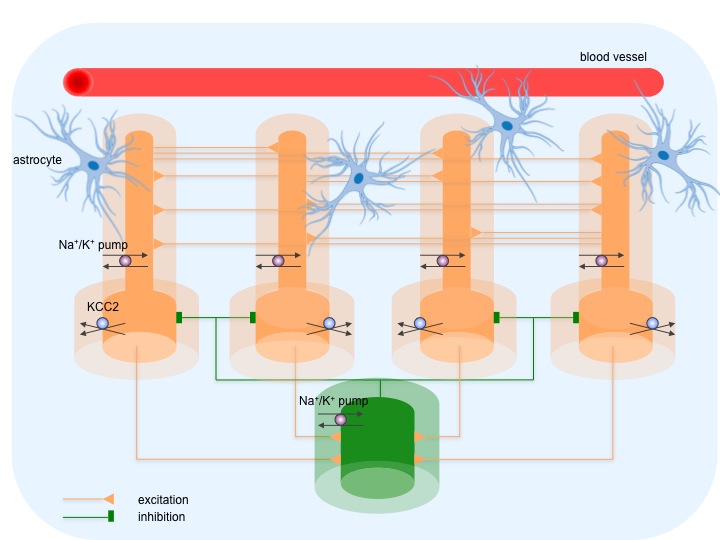
Figure 2. The model consists of five cells - four pyramidal cells (yellow) and an interneuron (green), which are synaptically coupled. Darker and lighter shade of the color corresponds to neurons and their enclosing extracellular compartments, surrounded by a bath (blue). The model includes ionic regulation mechanisms: Na+/K+ pump in both cells, KCC2 cotransporter in pyramidal cells, buffering of K+ ions by glial cells and vasculature and diffusion of K+, Na+ and Cl- ions between compartments and between extracellular space and surrounding bath.
The main insight obtained with the model is confirmation of experimentally derived novel hypothesis of seizure generation. Contrary to common and long-established view, these experimental data and our model simulation suggest that seizures may be initiated by strong discharges of inhibitory interneurons. They contribute to increase of potassium concentration in the extracellular space that results in neuronal depolarization and pathological discharges in the network of neurons.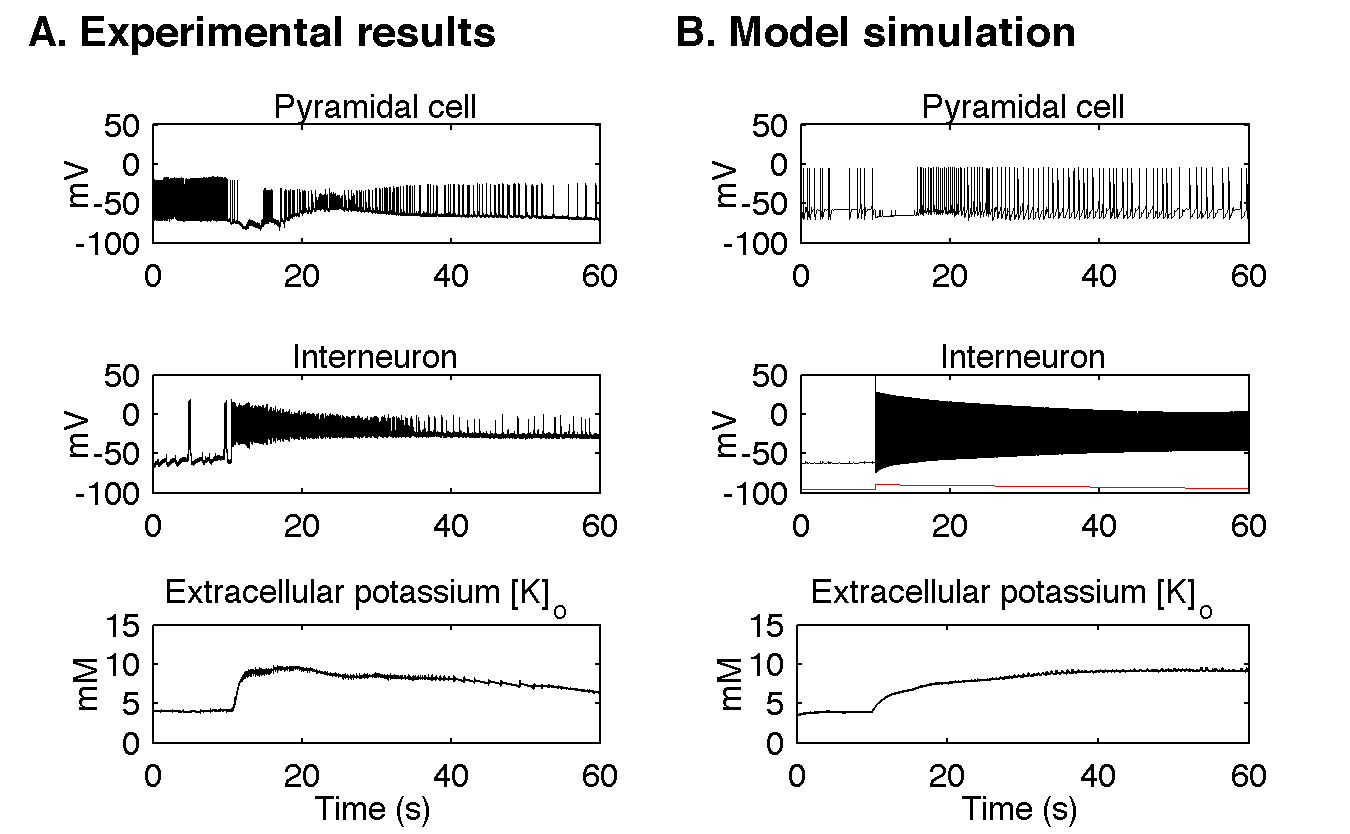
Figure 3. Comparison of experimental and modelling results. A. In the experimental data seizure activity starts with fast discharge of inhibitory interneurons (here around the time of 10 s). Pyramidal cells are transiently inhibited but after about 5 seconds they resume activity in the form of fast tonic spiking, which evolves towards intrinsic bursting behaviour. Extracellular potassium concentration attained increases sharply at the beginning of the seizure and stays elevated. To make the role of inhibitory interneurons more evident a positive current was delivered via the intracellular pipette to the pyramidal cell. The resulting depolarization was responsible for fast discharge present before the seizure. B. In the model the seizure-like event was initiated by depolarizing current applied to the inhibitory interneuron as shown by red trace in Figure 3B middle panel. Current stimulation triggered strong interneuronal discharge, which transiently inhibited pyramidal cell from firing. After few seconds pyramidal cell resumed its activity and generated tonic discharges, which evolved into bursts. All these changes were accompanied by increase of extracellular potassium concentration. It shows that simulation results agree qualitatively and quantitatively in many respects with the experimentally observed seizures in the isolated guinea pig brain.
From this scenario it follows that extracellular potassium accumulation plays an essential role in seizure generation. Hence, therapeutic interventions that would control the level of extracellular potassium could be effective at reducing or of even preventing seizures. To verify this hypothesis we added to the model additional potassium buffer, that could be possibly realized in practice by a nanoparticle system. Model simulation showed that despite strong interneuronal discharges, normal level of potassium concentration was immediately restored and seizure activity was prevented.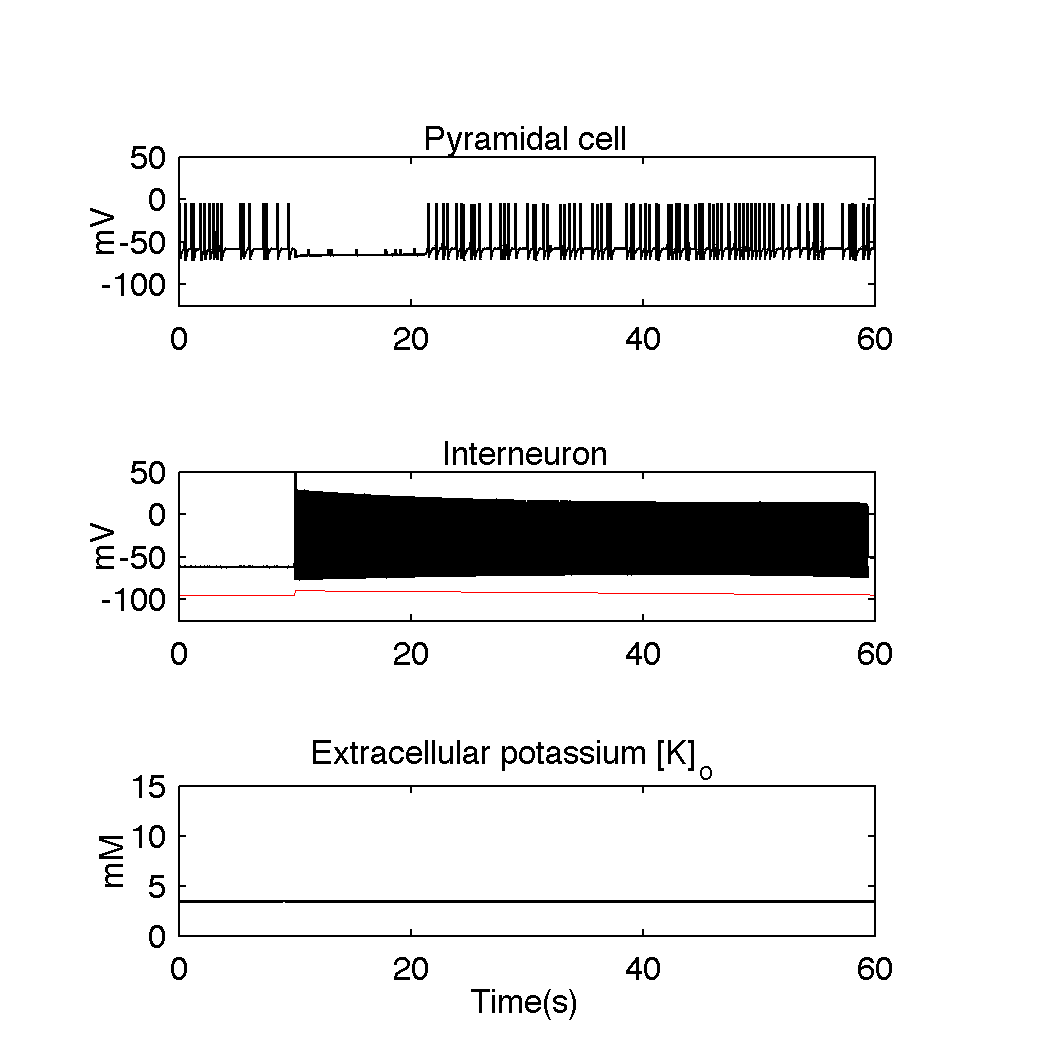
Figure 4. Model simulations with additional nanoparticle potassium buffering system present in the extracellular space. Despite strong discharge of the interneuron, extracellular potassium level does not increase and remains at its initial reference level. When GABAA mediated inhibition decreases around second 20, pyramidal cell exhibits normal firing. This simulation illustrates that artificial potassium buffering mechanisms could be effective in preventing seizure activity.

Figure 1. A. Whole guinea pig brain in vitro experimental setup. B. Brain in the aquarium in the sci-fi movie The City of the Lost Children (1995).
Currently, our computational model consists of five multicompartmental cells - four pyramidal neurons and an inhibitory interneuron - embedded in the common extracellular space surrounded by a bath, where concentrations are at their normal physiological levels. Neurons are connected by chemical excitatory (AMPA) and inhibitory (GABAA) synapses. Ionic dynamics of K+, Na+, Ca2+ and Cl− is incorporated and changes in their intracellular and extracellular concentrations are computed. Concentration changes are dependent on a number of mechanisms such as: active and passive membrane currents, inhibitory synaptic GABAA currents, Na+/K+ pump, KCC2 cotransporter, glial K+ buffering, radial diffusion between extracellular space and bath, longitudinal diffusion between dendritic and somatic compartments and volume changes of intra- and extracellular space.

Figure 2. The model consists of five cells - four pyramidal cells (yellow) and an interneuron (green), which are synaptically coupled. Darker and lighter shade of the color corresponds to neurons and their enclosing extracellular compartments, surrounded by a bath (blue). The model includes ionic regulation mechanisms: Na+/K+ pump in both cells, KCC2 cotransporter in pyramidal cells, buffering of K+ ions by glial cells and vasculature and diffusion of K+, Na+ and Cl- ions between compartments and between extracellular space and surrounding bath.
The main insight obtained with the model is confirmation of experimentally derived novel hypothesis of seizure generation. Contrary to common and long-established view, these experimental data and our model simulation suggest that seizures may be initiated by strong discharges of inhibitory interneurons. They contribute to increase of potassium concentration in the extracellular space that results in neuronal depolarization and pathological discharges in the network of neurons.

Figure 3. Comparison of experimental and modelling results. A. In the experimental data seizure activity starts with fast discharge of inhibitory interneurons (here around the time of 10 s). Pyramidal cells are transiently inhibited but after about 5 seconds they resume activity in the form of fast tonic spiking, which evolves towards intrinsic bursting behaviour. Extracellular potassium concentration attained increases sharply at the beginning of the seizure and stays elevated. To make the role of inhibitory interneurons more evident a positive current was delivered via the intracellular pipette to the pyramidal cell. The resulting depolarization was responsible for fast discharge present before the seizure. B. In the model the seizure-like event was initiated by depolarizing current applied to the inhibitory interneuron as shown by red trace in Figure 3B middle panel. Current stimulation triggered strong interneuronal discharge, which transiently inhibited pyramidal cell from firing. After few seconds pyramidal cell resumed its activity and generated tonic discharges, which evolved into bursts. All these changes were accompanied by increase of extracellular potassium concentration. It shows that simulation results agree qualitatively and quantitatively in many respects with the experimentally observed seizures in the isolated guinea pig brain.
From this scenario it follows that extracellular potassium accumulation plays an essential role in seizure generation. Hence, therapeutic interventions that would control the level of extracellular potassium could be effective at reducing or of even preventing seizures. To verify this hypothesis we added to the model additional potassium buffer, that could be possibly realized in practice by a nanoparticle system. Model simulation showed that despite strong interneuronal discharges, normal level of potassium concentration was immediately restored and seizure activity was prevented.

Figure 4. Model simulations with additional nanoparticle potassium buffering system present in the extracellular space. Despite strong discharge of the interneuron, extracellular potassium level does not increase and remains at its initial reference level. When GABAA mediated inhibition decreases around second 20, pyramidal cell exhibits normal firing. This simulation illustrates that artificial potassium buffering mechanisms could be effective in preventing seizure activity.
OUR TEAM
- Piotr Suffczyński
- Damiano Gentiletti
- Vadym Gnatkovsky (Fondazione I.R.C.C.S. Istituto Neurologico Carlo Besta)
- Marco de Curtis (Fondazione I.R.C.C.S. Istituto Neurologico Carlo Besta)